ARG20504
anti-Hsp 90 antibody
anti-Hsp 90 antibody for ELISA,ICC/IF,IHC-Formalin-fixed paraffin-embedded sections,Immunoprecipitation,Western blot and Human,Mouse,Rat
Cancer antibody; Signaling Transduction antibody
Overview
| Product Description | Rabbit Polyclonal antibody recognizes Hsp 90 |
|---|---|
| Tested Reactivity | Hu, Ms, Rat |
| Tested Application | ELISA, ICC/IF, IHC-P, IP, WB |
| Host | Rabbit |
| Clonality | Polyclonal |
| Target Name | Hsp 90 |
| Antigen Species | Human |
| Immunogen | Full length of Human Hsp90 protein (NP_031381.2). |
| Conjugation | Un-conjugated |
| Alternate Names | HSPC2; D6S182; Heat shock 84 kDa; HSP90B; HSP84; HSP 84; Heat shock protein HSP 90-beta; HSP 90; HSPCB |
Application Instructions
| Application Suggestion |
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Application Note | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
Properties
| Form | Liquid |
|---|---|
| Purification | Affinity purification with immunogen. |
| Buffer | Rabbit antiserum |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | HSP90AB1 |
| Gene Full Name | heat shock protein 90kDa alpha (cytosolic), class B member 1 |
| Background | Hsp90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. From a functional perspective, hsp90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex (1-4). Despite its label of being a heat-shock protein, hsp90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the hsp90-regulated proteins that have been discovered to date are involved in cell signaling (5-6). The number of proteins now know to interact with Hsp90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase.5. When bound to ATP, Hsp90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, hsp90-interacting proteins have been shown to co-precipitate with hsp90 when carrying out immunoadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in hsp90 expression or hsp90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit hsp90 function (7). 1. Arlander S.J.H., et al. (2003) J Biol Chem 278: 52572-52577. 2. Pearl H., et al. (2001) Adv Protein Chem 59:157-186. 3. Neckers L., et al. (2002) Trends Mol Med 8:S55-S61. 4. Pratt W., Toft D. (2003) Exp Biol Med 228:111-133. 5. Pratt W., Toft D. (1997) Endocr Rev 18: 306–360. 6. Pratt W.B. (1998) Proc Soc Exptl Biol Med 217: 420–434. 7. Whitesell L., et al. (1994) Proc Natl Acad Sci USA 91: 8324– 8328. |
| Function | Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. [UniProt] |
| Research Area | Cancer antibody; Signaling Transduction antibody |
| Calculated MW | 83 kDa |
| PTM | Ubiquitinated in the presence of STUB1-UBE2D1 complex (in vitro). ISGylated. S-nitrosylated; negatively regulates the ATPase activity. Phosphorylation at Tyr-301 by SRC is induced by lipopolysaccharide (PubMed:23585225). Phosphorylation at Ser-226 and Ser-255 inhibits AHR interaction (PubMed:15581363). Methylated by SMYD2; facilitates dimerization and chaperone complex formation; promotes cancer cell proliferation. Cleaved following oxidative stress resulting in HSP90AB1 protein radicals formation; disrupts the chaperoning function and the degradation of its client proteins. |
Images (3) Click the Picture to Zoom In
-
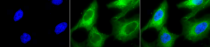
ARG20504 anti-Hsp 90 antibody ICC/IF image
Immunofluorescence: Heat Shocked (42°C for 1 hour) HeLa cells. Fixation: 2% Formaldehyde for 20 min at RT. Primary antibody: ARG20504 anti-Hsp 90 antibody at 1:100 for 12 hours at 4°C. Secondary antibody: FITC Goat anti-Rabbit (green) at 1:200 for 2 hours at RT. Counterstain: DAPI (blue) nuclear stain at 1:40000 for 2 hours at RT. Magnification: 100x. Left: DAPI (blue) nuclear stain. Middle: Primary antibody. Right: Composite.
-
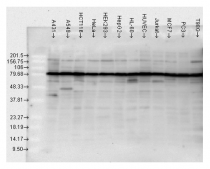
ARG20504 anti-Hsp 90 antibody WB image
Western blot: A431, A549, HCT116, HeLa, HEK293, HepG2, HL-60, HUVEC, Jurkat, MCF7, PC3, and T98G cell lysates stained with ARG20504 anti-Hsp 90 antibody.
-
ARG20504 anti-Hsp 90 antibody ICC/IF image
Immunofluorescence: Heat Shocked (42°C for 1 hour) HeLa cells. Fixation: 2% Formaldehyde for 20 min at RT. Primary antibody: ARG20504 anti-Hsp 90 antibody at 1:100 for 12 hours at 4°C. Secondary antibody: APC Goat anti-Rabbit (red) at 1:200 for 2 hours at RT. Counterstain: DAPI (blue) nuclear stain at 1:40000 for 2 hours at RT. Magnification: 20x. Left: DAPI (blue) nuclear stain. Middle: Primary antibody. Right: Composite.